Which Best Describes the Oxidizing Agent in This Reaction
Cl2aq 2Braq 2Claq Br2aq Bromine Br is the oxidizing agent because it gains an electron. Whereas oxidation state of Br is changing from -2 to -3 this means Br is gaining electrons therefore it is an oxidizing agent.
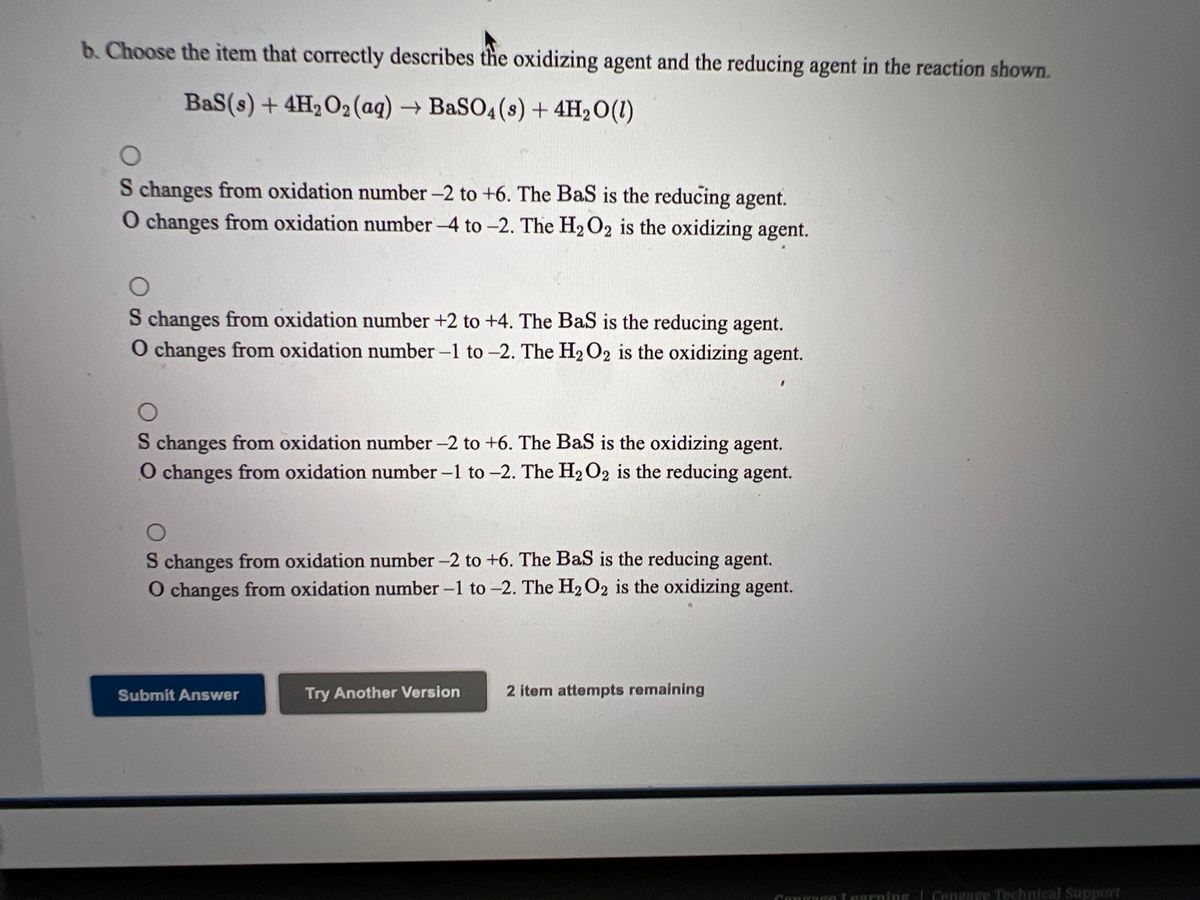
Answered A Choose The Item That Correctly Bartleby
In this chemical composition the oxidation state of the C atom is 3.

. Chlorine Cl is the oxidizing agent because it gains an electron. Which best describes the oxidizing agent in this reaction. In a neutral compound the sum of oxidation numbers is zero.
Hence it is oxidized and is acting as reducing agent. 17 Which of the following is an oxidation agent. Which of the following statements best describes an oxidizing agent.
Asked By Wiki User. A number that indicates how many electrons an atom has gained lost or shared to form a chemical bond with one or more atoms. Oxalic acid on the other hand is a reducing agent in this reaction.
By giving up electrons it reduces the MnO 4-ion to Mn 2. The strongest oxidizing agent in the list is F_2 followed by H_2O_2 and so on down to the. 15 Which best describes oxidation-reduction reactions.
You rank oxidizing agents according to their standard reduction potentials. Which of the following statements best describes an oxidizing agent. Bromine Br is the oxidizing agent because it loses an electron.
The substance M which itself gets oxidized reduces other and is called as reducing agent. Heres a typical table of standard reduction potentials. Oxidizing agent also known as oxidant gains electrons and it is reduced during chemical reaction.
Since we know H 2 C 2 O 4 is known to be a good reducing agent. It is a reactant that removes electrons from other reactants during a redox reaction. Cl₂ 2 e 2 Cl One molecule.
Which best describes an oxidizing agent. C A substance that is oxidized and causes reduction of another substance. An oxidizing agent is a reactant that removes electrons from other reactants during a redox reaction.
Common examples of oxidizing agents include halogens such as chlorine and fluorine oxygen and hydrogen peroxide H 2 O 2. Hydrogen peroxide ozone oxygen potassium nitrate and nitric acid are all oxidizing agents. It means a reactant which oxidizes other element or reactant ie it undergoes reduce themselves and gain an electron.
C A substance that is oxidized and causes reduction of another substance. An oxidizing agent often referred to as an oxidizer or an oxidant is a chemical species that tends to oxidize other substances ie. A A substance that is reduced and causes oxidation of another substanceb A substance that increases the number of oxygen atoms bonded.
Describe an oxidation reaction where the oxidation of a positive oxidation state increases over the reaction. One may also ask which best describes an oxidizing agent. Thus the MnO 4-ion acts as an oxidizing agent in this reaction.
A A substance that is reduced and causes oxidation of another substance. B A substance that increases the number of oxygen atoms bonded. The oxidizing agent can have two meanings.
Which best describes the oxidizing agent in this reaction. Oxidation agent is normally on its higher oxidation state since in gain electrons and be reduced. Cl2aq 2Braq 2Claq Br2aq Bromine Br is the oxidizing agent because it gains an electron.
A Electrons are being added to all substances Electrons are lost during oxidation and gained during reduction Electrons are being transferred from the oxidizing agent to the reducing agent Olons are forming as electrons are shared between metals and nonmetals. Which best describes the oxidizing agent in this reaction. 18 What is oxidized and what is reduced in the following reaction.
Bromine Br is the oxidizing agent because it loses an electron. Start studying the Oxidation-Reduction Quiz flashcards containing study terms like Which describes the oxidizing agent in a chemical reaction Given the reaction below which is the oxidized substance. A reaction in which electrons are transferred from one substance to another substance.
16 What describes the oxidizing agent in a chemical reaction. Memorize flashcards and build a practice test to quiz yourself before your exam. Atoms ions and molecules that have an unusually large affinity for electrons tend to.
It oxidizes the reducing agent. D A substance that reacts with oxygen. Cause an increase in the oxidation state of the substance by making it lose electrons.
The permanganate ion removes electrons from oxalic acid molecules and thereby oxidizes the oxalic acid. In a redox reaction the oxidizing agent is reduced. Which statement best describes what is happening in a redox reaction.
Mg Cl2 Mg2 2Cl Which best identifies why the rusting of an iron nail in the. Oxidizing is defined as.

Answered A Choose The Item That Correctly Bartleby

Answered A Choose The Item That Correctly Bartleby

Solved A Choose The Item That Correctly Describes The Chegg Com

Solved The Reaction Of Copper And Silver Nitrate Is Chegg Com
No comments for "Which Best Describes the Oxidizing Agent in This Reaction"
Post a Comment